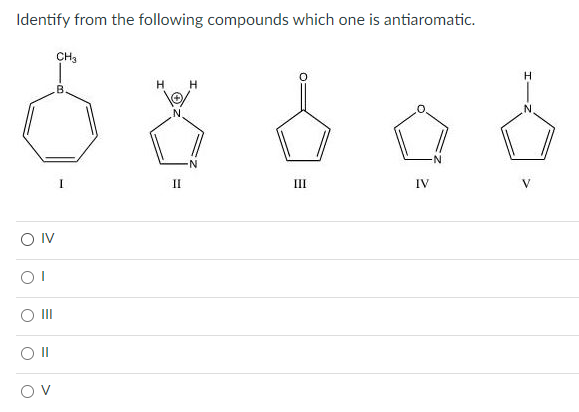
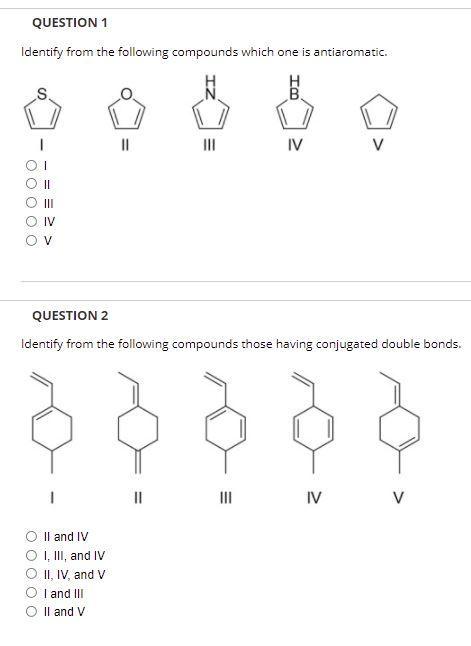
Identify From the Following Compounds Which One Is Antiaromatic.
Hello Today well be talking about Chapter 19 Question 36 which asks us about naming organic compounds. Pages 13 This preview shows page 9 - 13 out of 13 pages.
Solved Identify From The Following Compounds Which One Is Chegg Com
Which of the following is anti-aromatic in nature.
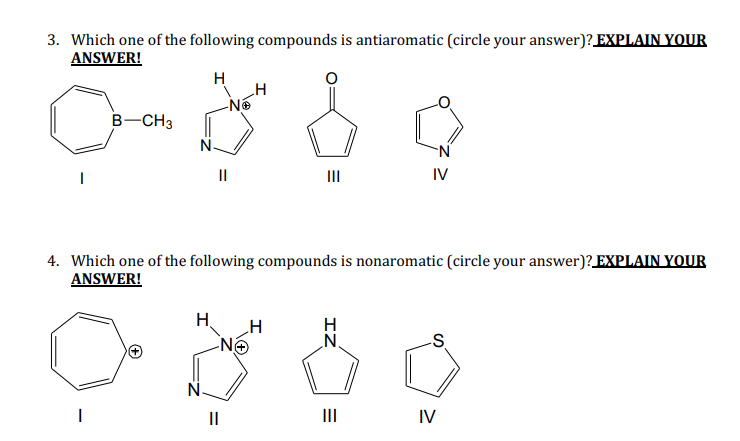
. Absorption at 3200-3600 cm-1 should disappear absorption at 3200-3600 cm-1 and 1100 cm-3 should disappear. From the above compounds which satisfy this condition is called the anti -aromatic compound. 100 1 rating Option.
I II III IV For the following reaction which of the following is consistent with the IR spectrum of the product. Identify from the following compounds which one is antiaromatic. Identify from the following compounds which one is aromatic.
II nonaromatic nonaromatic. 25 which one of the following compounds is. Mensuration Factorisation Linear Equations in One Variable Understanding Quadrilaterals The Making of the National Movement.
Of pi electrons 8. Which one of the following statements is not true for a compound to be considered as aromatic. A compound is said to the anti-aromatic if it is cyclic planar have the hybridization as s p 2 and obeys the 4 n π e s rule in which n can be any integer such as 123---and so on.
I-Z CH3 B IV V O III O IV OT OV O II For the following molecules classify them as aromatic antiaromatic or nonaromatic. It looks like a perfect student to be named as Anti-aromatic compound cyclic planer fully conjugated 8 pi electrons obeys 4n rule earlier I said that the case is different. HOH N I-Z Z-I N N N IV 1 II III ООООО III.
School Saint Marys College of California. сна H H H B N II III IV OIV 01 O II OV. HOH N I-Z Z-I N II MI III TV V - OOOO 11 111 IV V.
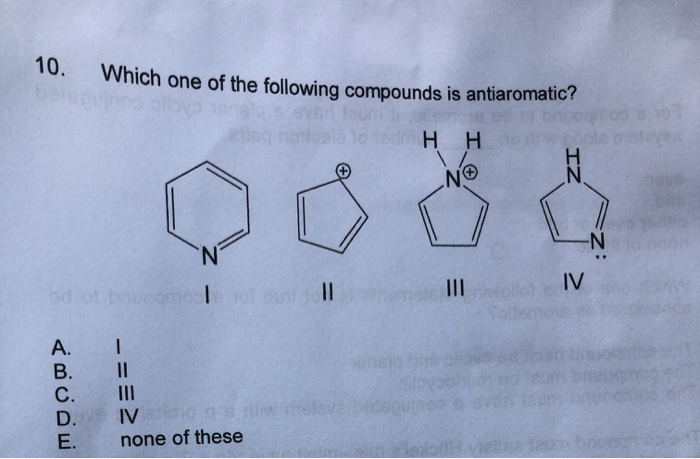
Class 9 Circles. I II III IV Which one of the following compound is nonaromatic. Experts are tested by Chegg as specialists in their subject area.
The compound must have a conjugated system with a p orbital at every vertex D. Antiaromaticity is a characteristic of a cyclic molecule with a π electron system that has higher energy due to the presence of 4n delocalised π or lone pair electrons in it. The above compound is an aromatic compound.
I 11 IV V O II O IV OT O OV Identify from the following compounds which one is nonaromatic. Course Title CHM 2020. II antiaromatic 1 aromatic.
Who are the experts. Next will count up the carbon chain giving this functional group the lowest numbers possible. To avoid the instability of antiaromaticity.
It is also important to note that Huckels Rule is just one of three main rules in identifying an aromatic compound. I mean for Al Keen. Chemistry questions and answers.
A good example is cyclooctatetraene which we have talked about earlier. Unlike aromatic compounds which follow Hückels rule 4n2 π electrons and are highly stable antiaromatic compounds are highly unstable and highly reactive. The third and sixth compounds are planar and fully conjugated but they do not satisfy the Huckels rule in the third case no.
View the full answer. Identify from the following compounds which one is antiaromatic. Il aromatic O I aromatic.
V and VI both have 4e and planar structure. 0 II III IV OV OT O II O III O IV Identify from the following compounds which one is antiaromatic. We review their content and use your feedback to keep the quality high.
The compound must be monocyclic. Click hereto get an answer to your question Which of the following is anti - aromatic compound. Cyclooctatetraene is showing some extra behaviour to avoid Anti-aromaticity.
Anti-aromatic compound contains 4nπ electrons. This gives us the Suffolk CE. Up to 10 cash back For example 402 gives a two-pi-electron aromatic compound.
Identify from the following compounds which one is antiaromatic. The compound must be cyclic and planar B. HOH N I-Z Z-I N II MI III TV V - OOOO 11 111 IV V.
Anti Aromatic compounds are those compounds which satisfy the rules of planarity and fully conjugation of the pi electrons inside the ring but fail to satisfy Huckels rule of 4n2 pi electrons. An annulene is a system of conjugated monocyclic hydrocarbons. Pyridine is less basic than triethylamine because AIIMS-2005 1 Pyridine has aromatic character 2 Nitrogen in pyridine is sp 2 hybridized 3 Pyridine is a cyclic system 4 In pyridine lone pair of nitrogen is delocalized.
And so here well start by looking at the functional group of the molecule. 11 antiaromatic O I. View the full answer.
Correct option is B Option B is the correct answer. A compound is considered anti-aromatic if it follows the first two rules for aromaticity 1. The compound must satisfy Hückels rule -must have 4n 2 electrons.
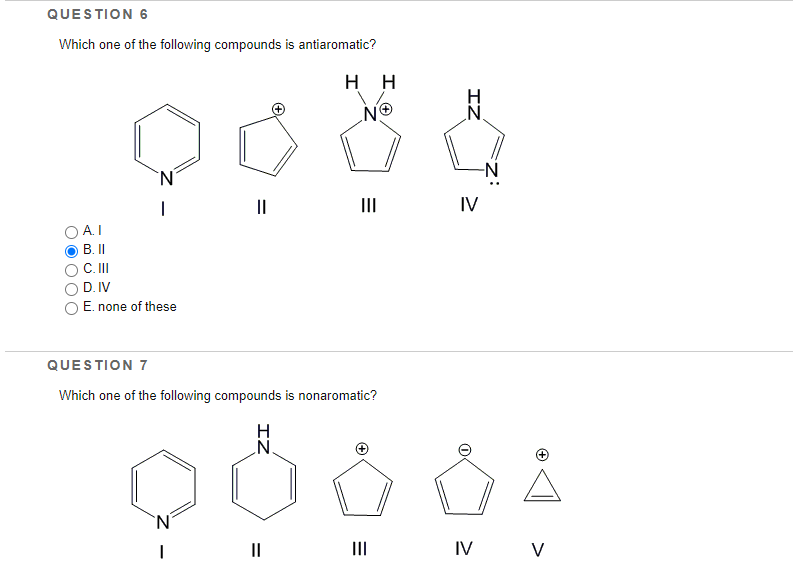
25 Which one of the following compounds is antiaromatic A I B II C III D IV E. An Excellent example for this is Cyclooctatetraene. The main functional group is the AL Keen here.
Identify from the following compounds which one is aromatic. 加工 II I nonaromatic. Complete step by step answer.
Have 4n2 πe and system should be planar where n is an integer. It looks like a perfect candidate to be named antiaromatic cyclic planar fully conjugated and 8 electrons 4n formula for antiaromatic compounds. Which one of the following compound is antiaromatic.
Which one of the following compounds is most acidic -AIPMT-2005 1 OH NO 2 2 OH CH 3 3 OH 4 ClCH 2 CH 2 OH 9. Join Login Class 11 Chemistry Hydrocarbons.
Solved Question 6 Which One Of The Following Compounds Is Chegg Com
Solved 10 Which One Of The Following Compounds Is Chegg Com
Solved Question 1 Identify From The Following Compounds Chegg Com
Comments
Post a Comment